业内动态
cGMP制药洁净室整体解决方案:细胞治疗产品、单克隆抗体、疫苗以及口服固体抗病毒药物的生产
发布时间:2022-04-08 17:23:12
● ● 齐 心 协 力 | 共 渡 难 关 ● ●

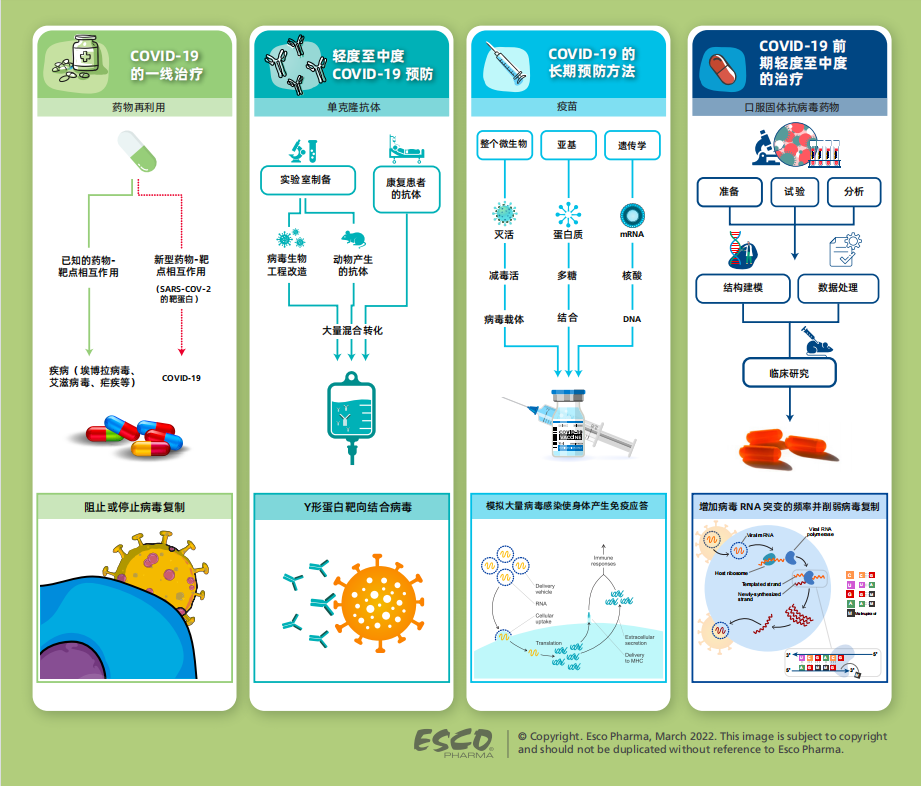
目前,全球仍在继续竞相寻找抗击COVID-19 爆发流行的最有效策略。迫切需要有效的公共卫生干预措施和可靠的治疗手段来扭转这一激增趋势,与此同时也推动了多种用于改善患者预后的药物开发。
感染早期进行治疗是最有希望的,在寻找早期重要的临床数据过程中,发现当前批准的药物有新的适应症(又称药物再利用)。基于这个情况,把早期治疗中,对人体安全有效的药物分子进行联合用药,用于各种筛选试验。据说这是一种具有成本效益的药物开发技术,相比新疗法可以更快地治疗新冠肺炎患者。
人类与COVID-19的斗争仍在继续,从长远角度来看,接种新冠疫苗是最有效的预防手段,它有助于个体产生免疫力,在不良事件发生时使个人感染风险降到最低。疫苗是一种生物物质,在外来入侵物质(如病毒)刺激身体后做出应答并产生抗体。抗体是由免疫B细胞天然产生的蛋白质,其主要机制是通过与病毒部分特异性结合并阻止其进入细胞,从而免于感染或者控制感染发展为疾病。大多数获得批准的疫苗基本是通过皮下注射和肌肉注射这两种方式进行接种。

通常,这些抗体在接种疫苗或感染后自然产生,但也可以在实验室中通过生物工程进行制备。实验室制备的抗体称为单克隆抗体 (mAb),其产生的方式主要通过静脉注射以及注射给药。
尽管最近全球疫情有所改善,但许多国家仍面临着早期治疗需求无法满足的困境,早期检测对于避免产生重症病例和免于住院治疗具有重要意义。因此,随着治疗方法的不断研究,抗病毒的口服固体制剂(OSD)出现并应用。第一个用于早期COVID-19治疗的抗病毒口服药物是由默克公司研发的莫努匹韦,临床数据表明,在治疗初期或病毒仍在复制的阶段,该药品具有很高的治疗疗效,这种药物会增加病毒RNA突变的频率并削弱病毒复制。




参考文献:
1. Brande, D., Milholland, D., & Haycocks, N. (2017). Why is 90 FPM Considered Standard for Cleanroom Airflow?. ISPE. Retrieved from: https://ispe.org/pharmaceutical-engineering/march-april-2017/why-90-fpm-considered-standard-cleanroom-airflow
2. Channel News Asia (2021). Merck COVID-19 pill sparks calls for access for lower income countries. Retrieved from: https://www.channelnewsasia.com/world/merck-covid-19-pill-sparks-calls-access-lower-income-countries-2250266
3. COVID-19 Prevention Network. (n.d.). The Science of COVID-19 Vaccines and Antibodies. Retrieved from: https://www.coronaviruspreventionnetwork.org/coronavirus-vaccine-and-antibody-science/
4. Cumbers, John (2020). Timeline Shows 3 Paths To COVID-19 Treatment And Prevention (Infographics). Forbes. Retrieved from: https://www.forbes.com/sites/johncumbers/2020/03/25/timeline-shows-3-paths-to-COVID-19-treatment-and-prevention-infographic/?sh=25e975ad4789
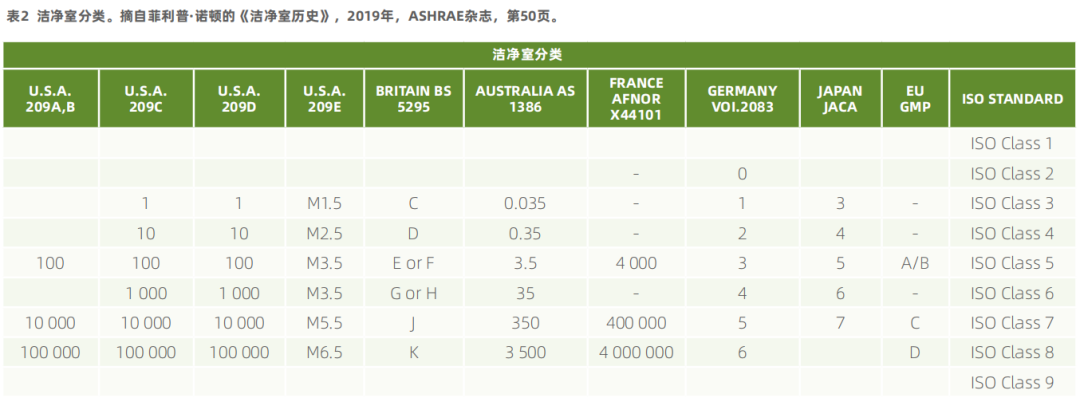
5. Institute of Environmental Sciences and Technology. (2001). FED-STD-209E. Retrieved from: https://www.iest.org/Standards-RPs/ISO-Standards/FED-STD-209E
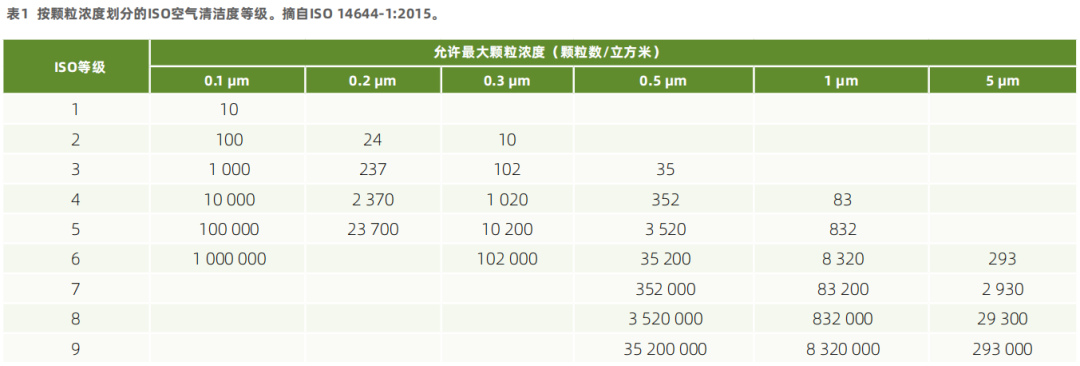
6. International Organization for Standardization. (2015). ISO 14644-1:2015, Cleanrooms and associated environments - Part 1: Classification of air cleanliness by particle concentration (2nd ed.). ISO 2015.
7. Naughton, P. (2019). History of Cleanrooms. ASHRAE Journal. Retrieved from: https://www.ashrae.org/file%20library/technical%20resources/ashrae%20journal/125thanniversaryarticles/38-54_naughton_historical_v2.pdf
8. Ramirez, J., Sharpe, L., & Peppas, N. (2017). Current state and challenges in developing oral vaccines. Advanced Drug Delivery Reviews, 114, 116-131. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6132247/
9. U.S. Department of Health and Human Services. (2004). Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice. Food and Drug Administration. Retrieved from: https://www.fda.gov/media/71026/download
10. Vaccines Europe. (2020). How are vaccines produced? Retrieved from: https://www.vaccineseurope.eu/about-vaccines/how-are-vaccines-produced
图文crESCO生命科学、网络
- E N D -